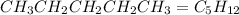Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 02:30
24 points and brainliest to anyone who can answer under 10 minutes with best ! the table below shows the role of different substances during photosynthesis. substance role during photosynthesis glucose stores chemical energy water combines with glucose to form carbon dioxide chlorophyll traps sunlight which of the following statements would correct one of the roles listed in the table? glucose combines with carbon to form water. chlorophyll reacts with light to produce carbon dioxide. water combines with carbon dioxide during photosynthesis. chlorophyll stores chemical energy needed for photosynthesis.
Answers: 1

Chemistry, 22.06.2019 07:30
The table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 3

Chemistry, 22.06.2019 12:40
Consider the directing effects of the substituents on salicylamide and predict the possible structures of the iodination products. which do you think will be the major product?
Answers: 1

Chemistry, 22.06.2019 18:00
To apply in a gold the individual gold atoms are united to each other by means of a metallic bond. how would you use the gold block to determine the atomic radius of a gold atom?
Answers: 3
You know the right answer?
What is the total mass of hydrogen in each of the molecules?
(a)ch4
(b)chcl3
(c)...
(a)ch4
(b)chcl3
(c)...
Questions




Mathematics, 08.02.2021 23:30

History, 08.02.2021 23:30





Mathematics, 08.02.2021 23:30

Mathematics, 08.02.2021 23:30

Mathematics, 08.02.2021 23:30

Mathematics, 08.02.2021 23:30

Mathematics, 08.02.2021 23:30


Arts, 08.02.2021 23:30


Biology, 08.02.2021 23:30

History, 08.02.2021 23:30

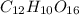 there are 10 moles of hydrogen atom
there are 10 moles of hydrogen atom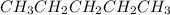 there are 12 moles of hydrogen atom.
there are 12 moles of hydrogen atom.