
Chemistry, 02.09.2019 16:20 Justus4215
Areaction a(aq)+b(aq)↽−−⇀c(aq) has a standard free‑energy change of −4.20 kj/mol at 25 °c. what are the concentrations of a, b, and c at equilibrium if, at the beginning of the reaction, their concentrations are 0.30 m, 0.40 m, and 0 m, respectively?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 07:00
What is the main purpose of patent attorneys? defend the company against legal claims manage financial investments invent new products protect rights to new products and processes
Answers: 1

Chemistry, 22.06.2019 10:10
What shape would a molecule with two bound groups and two lone pairs have?
Answers: 1

Chemistry, 23.06.2019 04:31
Areaction is first order. if the initial reactant concentration is 0.0200 m, and 25.0 days later the concentration is 6.25 x 10-4 m, then its half-life is:
Answers: 1

Chemistry, 23.06.2019 07:40
Which of the following has expanded our knowledge of the universe beyond our solar system the most? a. manned space travel b. the hubble space telescope c. the pioneer and voyager missions d. the international space station
Answers: 3
You know the right answer?
Areaction a(aq)+b(aq)↽−−⇀c(aq) has a standard free‑energy change of −4.20 kj/mol at 25 °c. what are...
Questions

Computers and Technology, 15.12.2020 23:40



English, 15.12.2020 23:40


Mathematics, 15.12.2020 23:40



English, 15.12.2020 23:40

English, 15.12.2020 23:40



Mathematics, 15.12.2020 23:40




English, 15.12.2020 23:40

World Languages, 15.12.2020 23:40

History, 15.12.2020 23:40

Mathematics, 15.12.2020 23:40

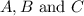 at equilibrium are 0.132 M, 0.232 M and 0.168 M respectively.
at equilibrium are 0.132 M, 0.232 M and 0.168 M respectively.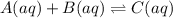
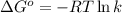
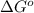 = standard free‑energy change = -4.20 kJ/mole
= standard free‑energy change = -4.20 kJ/mole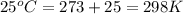
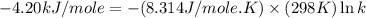
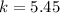
![k=\frac{[C]}{[A][B]}](/tpl/images/0220/8877/b93eb.png)
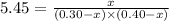
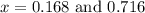
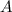 at equilibrium = (0.30-x) = 0.30 - 0.168 = 0.132 M
at equilibrium = (0.30-x) = 0.30 - 0.168 = 0.132 M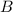 at equilibrium = (0.40-x) = 0.40 - 0.168 = 0.232 M
at equilibrium = (0.40-x) = 0.40 - 0.168 = 0.232 M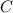 at equilibrium = x = 0.168 M
at equilibrium = x = 0.168 M

