Chemistry, 02.09.2019 16:30 carlinryan
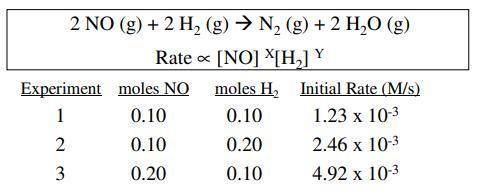
The reaction of nitric oxide with hydrogen at 1280 c is 2no(g) +2h2(g)n2(g)+2h2o(g) from the following data collected at this temperature, determine the rate law and calculate the rate constant. initial rate (m/s) 1.3 x 10 5.0 x 10 experiment 1 2 3 no 0050 0100 0100 .0020 .0020 0040 10.0 x 105

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 18:50
Question 3(multiple choice worth 4 points) (04.04 lc) what does it mean when an element is reduced? it empties a valance shell, reducing its atomic radius. it gains electrons, reducing its overall charge. it increases electronegativity, reducing its ability to bond. it loses electrons, reducing its electron number.
Answers: 1

Chemistry, 22.06.2019 22:00
The diagrams to the right show the distribution and arrangement of gas particles in two different containers. according to kinetic-molecular theory, which of the following statements is true? check all that apply. if the temperatures of both containers are equal, container a has greater pressure than container b. if the volume of container a decreased, its pressure would decrease. if the pressure in both containers is equal, container a has a lower temperature than container b.
Answers: 2


Chemistry, 23.06.2019 07:30
The compound formed from 2 atoms of hydrogen and one atom of oxygen is
Answers: 1
You know the right answer?
The reaction of nitric oxide with hydrogen at 1280 c is 2no(g) +2h2(g)n2(g)+2h2o(g) from the followi...
Questions









English, 15.04.2020 20:11


Mathematics, 15.04.2020 20:12