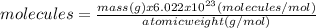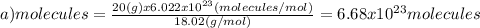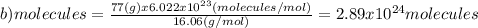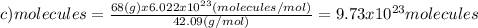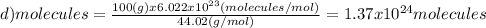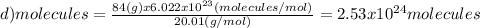Which of the following represents the least number of molecues?
(a)20.0 g of h2o (18.02g/mol)...

Chemistry, 02.09.2019 17:30 angelrgomez01
Which of the following represents the least number of molecues?
(a)20.0 g of h2o (18.02g/mol)
(b)77.0 g of ch4 (16.06 g/mol)
(c)68.0 g of cah2 (42.09 g/mol)
(d)100.0 g of n2o (44.02 g/mol)
(e)84.0 g of hf (20.01 g/mol)

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 01:00
What are the variables in gay-lussac’s law? pressure and volume pressure, temperature, and volume pressure and temperature volume, temperature, and moles of gas
Answers: 1

Chemistry, 22.06.2019 15:00
Which are forms of frozen water? check all that apply. dew frost hail rain sleet
Answers: 2

Chemistry, 22.06.2019 16:50
Ajet plane is speeding down the runway during takeoff. air resistance is not negligible. identify the forces on the jet.
Answers: 3

Chemistry, 23.06.2019 06:00
Each step in the following process has a yield of 70% ch4 + 4cl2 yield ccl4 +4hcl ccl4 + 2hf yield ccl2f2 + 2hcl of 4.50 mole ch4 reacts what is the total amount of hcl produced
Answers: 3
You know the right answer?
Questions


English, 18.11.2020 22:40






Mathematics, 18.11.2020 22:40

Mathematics, 18.11.2020 22:40

Physics, 18.11.2020 22:40

Mathematics, 18.11.2020 22:40


Mathematics, 18.11.2020 22:40


Geography, 18.11.2020 22:40


Mathematics, 18.11.2020 22:40

Mathematics, 18.11.2020 22:40

Arts, 18.11.2020 22:40