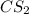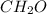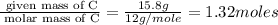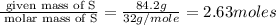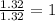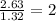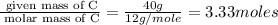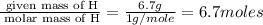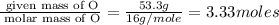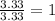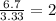Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 00:30
If 3.00 g of titanium metal is reacted with 6.00 g of chlorine gas, cl2, to form 7.7 g of titanium (iv) chloride in a combination reaction, what is the percent yield of the product?
Answers: 1

Chemistry, 22.06.2019 12:00
What is a possible quantum number set for an electron in the 3s orbital of a magnesium atom
Answers: 1

Chemistry, 22.06.2019 15:30
Which suspect most likely committed the robbery and how do you know
Answers: 2

Chemistry, 22.06.2019 16:50
Assuming complete dissociation of the solute, how many grams of kno3 must be added to 275 ml of water to produce a solution that freezes at -14.5 c? the freezing point for pure water is 0.0 c and k_f is equal to 1.86 c/m
Answers: 3
You know the right answer?
Determine the empirical formulas for compounds with the following percent compositions:
(a)15...
(a)15...
Questions




History, 25.11.2019 06:31


Chemistry, 25.11.2019 06:31


Mathematics, 25.11.2019 06:31

Mathematics, 25.11.2019 06:31


Mathematics, 25.11.2019 06:31

History, 25.11.2019 06:31



History, 25.11.2019 06:31

Mathematics, 25.11.2019 06:31

Biology, 25.11.2019 06:31

Mathematics, 25.11.2019 06:31


Mathematics, 25.11.2019 06:31