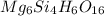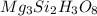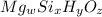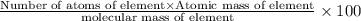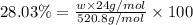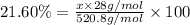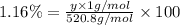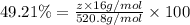Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 14:10
Amonoprotic acid is an acid that donates a single proton to the solution. suppose you have 0.140 g of a monoprotic acid dissolved in 35.0 ml of water. this solution is then neutralized with 14.5 ml of 0.110 m naoh. what is the molar mass of the acid?
Answers: 1

Chemistry, 22.06.2019 09:00
Which two scientific disciplines are bridged by pharmaceutical drugs? chemistry and forensics chemistry and medicine biology and forensics biology and criminology
Answers: 3

Chemistry, 22.06.2019 15:00
Areaction is first order with respect to reactant x and second order with respect to reactant y. which statement describes the rate law for this reaction?
Answers: 1

Chemistry, 22.06.2019 18:50
At stp, which substance is the best conductor of electricity? a. nitrogen b. neon c. sulfur d. silver
Answers: 1
You know the right answer?
Determining the empirical and molecular formula for chyrsotile asbestos. chyrsotile has the followin...
Questions

Mathematics, 22.11.2020 23:30








Mathematics, 22.11.2020 23:30

History, 22.11.2020 23:30




Mathematics, 22.11.2020 23:30



Mathematics, 22.11.2020 23:30

Mathematics, 22.11.2020 23:30