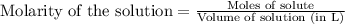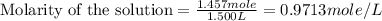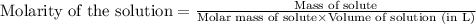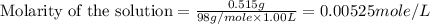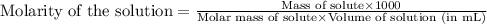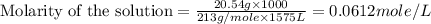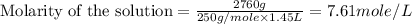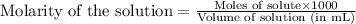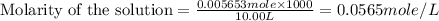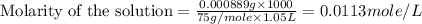Chemistry, 02.09.2019 18:20 aubreyfoster
Determine the molarity for each of the following solution solutions:
(a)1.457 mol of kcl in 1.500 l of solution
(b) 0.515 gram ofh2so4, in 1.00 l of solution
(c) 20.54 g of al(no3)3 in 1575 ml of solution
(d)2.76 kg ofcuso4.5h2o in 1.45 l of solution
(e)0.005653 mol ofbr2 in 10.00 ml of solution
(f) 0.000889 g of glycine, c2h5no2, in 1.05 ml of solution

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 21:30
An atomic nucleus is composed ofa)protons.b)protons and neutrons.c)protons and electrons.d)protons, neutrons, and electrons.
Answers: 1

Chemistry, 22.06.2019 23:30
Rank substituents in order of their priority when assigning the e or z label to an alkene. i, ch2i , h, ch2ch2cl, f
Answers: 2

Chemistry, 23.06.2019 00:30
Five different substances are given to you to be dissolved in water. which substances are most likely to undergo dissolution in water? check all that apply. view available hint(s) check all that apply. sodium fluoride, naf octane, c8h18 propanol, ch3ch2ch2oh potassium iodide, ki benzene, c6h6
Answers: 1
You know the right answer?
Determine the molarity for each of the following solution solutions:
(a)1.457 mol of kcl in...
(a)1.457 mol of kcl in...
Questions

Biology, 28.08.2020 14:01

Mathematics, 28.08.2020 14:01


Mathematics, 28.08.2020 14:01



Health, 28.08.2020 14:01





Chemistry, 28.08.2020 14:01

Mathematics, 28.08.2020 14:01




Business, 28.08.2020 14:01

History, 28.08.2020 14:01

Mathematics, 28.08.2020 14:01

Mathematics, 28.08.2020 14:01

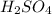 solution is, 0.00525 mole/L
solution is, 0.00525 mole/L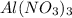 solution is, 0.0612 mole/L
solution is, 0.0612 mole/L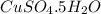 solution is, 7.61 mole/L
solution is, 7.61 mole/L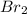 solution is, 0.0565 mole/L
solution is, 0.0565 mole/L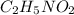 solution is, 0.0113 mole/L
solution is, 0.0113 mole/L