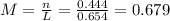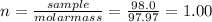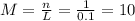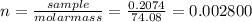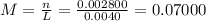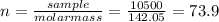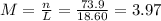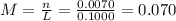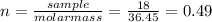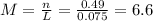Chemistry, 02.09.2019 18:20 nyraimccall408
Determine the molarity for each of the following solution solutions:
(a)0.444 mol of cocl2 in 0.654 l of solution
(b) 98.0 gram of phosphoric acid, h3po4, in 100 l of solution
(c) 0.2074 g of calcium hydroxide, ca(oh)2 in 40.00 ml of solution
(d)10.5 kg of na2ao4.10h2o in 18.60 l of solution
(e) 7.0 x 10-3 mol of l2 in 100.0 ml of solution
(f) 1.8 x 104 mg of hcl in 0.075 of solution

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 05:30
Liv sheldon given the balanced equation for an organic reaction: c2h2 + 2cl2 → c2h2cl4 this reaction is best classified as *
Answers: 1


Chemistry, 22.06.2019 20:20
Which formula equation represents the burning of sulfur to produce sulfur dioxide? s(s) + o2(g) 4502(9) 2h2s(s) + 302(g) —> 2h20(0) + 2502(9) 4fes2+1102 —> 2fe2o3 + 8502 2802(g) + o2(9) v205 , 2503(9)
Answers: 1
You know the right answer?
Determine the molarity for each of the following solution solutions:
(a)0.444 mol of cocl2 i...
(a)0.444 mol of cocl2 i...
Questions

English, 18.03.2021 01:50


Mathematics, 18.03.2021 01:50




Biology, 18.03.2021 01:50

Mathematics, 18.03.2021 01:50

Spanish, 18.03.2021 01:50

Mathematics, 18.03.2021 01:50


Mathematics, 18.03.2021 01:50



Mathematics, 18.03.2021 01:50





Mathematics, 18.03.2021 01:50