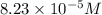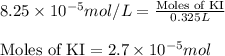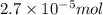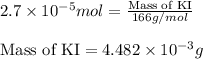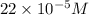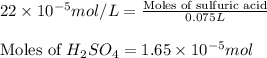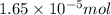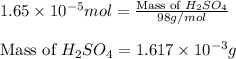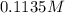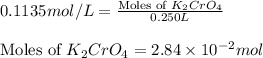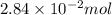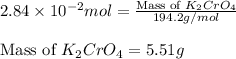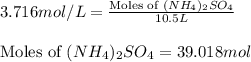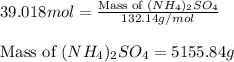Calculate the number of moles and the mass of the solute in each of the following solutions:
(a) 325 ml of 8.23 x 10-5 m kl, a source of iodine in the diet
(b) 75.0 ml of 22 x 10-5 m h2so4, a sample of acid rain
(c) 0.2500 l of 0.1135 m k2cro4 and analytical reagent used in iron assays
(d)10.5 l of 3.716 m (nh4)2so4, a liquid fertilizer

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 02:00
Will give brainliest it is a lab from k12 here is the linkfor each metal that participated in a chemical change, write the type of metal it is, based on your examination of the periodic table. type your answer here. (score for question 3: of 5 points) were there any metallic compounds that did not react with either the acid or the base? write the type of metal, based on your examination of the periodic table. type your answer here. (score for question 4: of 5 points) make a general statement about the reactivity of the metals in this experiment. type your answer here.
Answers: 2

Chemistry, 22.06.2019 10:30
Asample of air with a volume of 2.20m3 at a pressure of 105 kpa and a temperature of 30c is cooled to 10c and the pressure is reduced to 75.0 kpa. what is the new volume? 6.9 1.34 2.56 43.0 2.88
Answers: 1

Chemistry, 22.06.2019 18:00
The fact that the total amount of energy in a system remains constant is a(n)
Answers: 1

Chemistry, 22.06.2019 23:10
Using the periodic table, complete the following. element: hydrogen symbol: h₂ molecular weight: g mass of one mole: g/mol
Answers: 3
You know the right answer?
Calculate the number of moles and the mass of the solute in each of the following solutions:
...
...
Questions

Mathematics, 02.03.2021 19:30





Biology, 02.03.2021 19:30

Geography, 02.03.2021 19:30



History, 02.03.2021 19:30


Mathematics, 02.03.2021 19:30

Spanish, 02.03.2021 19:30

History, 02.03.2021 19:30

Mathematics, 02.03.2021 19:30

Mathematics, 02.03.2021 19:30


Chemistry, 02.03.2021 19:30

Spanish, 02.03.2021 19:30

History, 02.03.2021 19:30

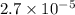 and mass is
and mass is 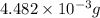
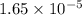 and mass is
and mass is 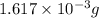
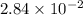 and mass is 5.51 g.
and mass is 5.51 g.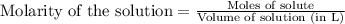 .....(1)
.....(1)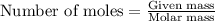 .....(2)
.....(2)