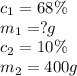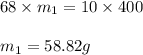Chemistry, 02.09.2019 19:10 DaylaReevaFEEVA2757
Consider this question: what mass of concentrated solution nitric acid( 68.0% hno3 by mass) is needed to prepare 400.0 g of a 10.0% solution of hno3 by mass?
(a) outline the steps necessary to answer the question
(b) answer the question

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 04:00
Tin has ten stable isotopes. the heaviest, 124sn, makes up 5.80% of naturally occuring tin atoms. how many atoms of 124sn are present in 82.0 g of naturally occurring tin? what is the total mass of the 124sn atoms in this sample?
Answers: 3

Chemistry, 22.06.2019 10:00
Miner's coal distributors does not mine coal itself, nor does it even store or handle the coal. instead, miner's solicits orders for low sulfur coal from other firms, then purchases the required amount from suppliers and directs them to ship the coal to its customers. what is miner's
Answers: 1

Chemistry, 22.06.2019 10:30
If you add 5.00 ml of 0.100 m sodium hydroxide to 50.0 ml of acetate buffer that is 0.100 m in both acetic acid and sodium acetate, what is the ph of the resulting solution? acetic acid: ka = 1.8. x 10-5
Answers: 1

You know the right answer?
Consider this question: what mass of concentrated solution nitric acid( 68.0% hno3 by mass) is need...
Questions

Mathematics, 08.10.2021 16:10


Mathematics, 08.10.2021 16:10


Mathematics, 08.10.2021 16:10

Mathematics, 08.10.2021 16:10

Mathematics, 08.10.2021 16:10



Business, 08.10.2021 16:10




History, 08.10.2021 16:10



Mathematics, 08.10.2021 16:10



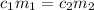
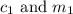 are the concentration and mass of concentrated solution.
are the concentration and mass of concentrated solution.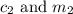 are the concentration and mass of diluted solution.
are the concentration and mass of diluted solution.