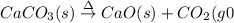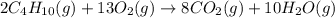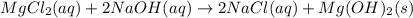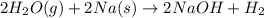Chemistry, 02.09.2019 20:10 codyshs160
Write a balanced molecular equation describing each of the following chemical reactions:
(a)solid calcium carbonate is heated and decomposes to sold calcium oxide and carbon dioxide
(b)gaseous butane, c4h10, reacts with diatomic oxygen gas to yield gaseous carbon dioxide and water vapor
(c)aaqeous solutions of magnesium chloride and sodium hydroxide react to produce solid magnesium hydroxide and aqueous sodium chloride
(d)water vapor reacts with sodium metal to produce solid sodium hydroxide and hydrogen gas

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 17:00
Reduction is a reaction which results in a in electrons and a in positive charge of the atom or ion 1) a- loss 1) b- gain 2) a-increase 2) b-decrease
Answers: 1


Chemistry, 23.06.2019 03:00
You have a sample of a metal, the sample is exactly 6.02 x 1023atom, if the sample has a mass 55.85 what metal is your sample made of?
Answers: 2

Chemistry, 23.06.2019 04:40
6) (a) calculate the absorbance of the solution if its concentration is 0.0278 m and its molar extinction coefficient is 35.9 l/(mol cm). the depth of the cell is 5 mm. (b) what is the %t? (7) calculate the absorbance of the solution if the transmitted light intensity is 70% of the initial light beam intensity
Answers: 1
You know the right answer?
Write a balanced molecular equation describing each of the following chemical reactions:
(a)s...
(a)s...
Questions


Mathematics, 17.11.2020 18:00

Mathematics, 17.11.2020 18:00

Mathematics, 17.11.2020 18:00



Mathematics, 17.11.2020 18:00






History, 17.11.2020 18:00


English, 17.11.2020 18:00


Biology, 17.11.2020 18:00

Mathematics, 17.11.2020 18:00