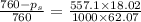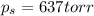Chemistry, 02.09.2019 20:20 genyjoannerubiera
Calculate the vapor pressure lowering of a solution at 100.0 °c that contains 557.1 g of ethylene glycol (molar mass g/mol). the vapor pressure of pure water at t00.0 °c is 760 torr. (2 points) 62.07 g/mol) in 1000.0 g of water (h20 molar mass 18.02 a) 106 torr b) 0.756 torr c) 760 torr (d)186 torr e) none of these

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 04:20
Which of the following is true for the actual yield of a reaction? it is always calculated as a ratio. it is the yield from the excess reactant. it is the yield from the limiting reactant. it is always less than the theoretical yield.
Answers: 1

Chemistry, 22.06.2019 06:30
Suppose a lab group reports a ppercent yield of sand of 105. is it really possible to collect more sand than was originally represented? what is the possible explanation for the extra product?
Answers: 2


Chemistry, 22.06.2019 15:00
20 pts ‼️ an unmanned spacecraft travels to mars. mars has a lower strength of gravity than earth. where in the image is the spacecraft’s weight the greatest?
Answers: 2
You know the right answer?
Calculate the vapor pressure lowering of a solution at 100.0 °c that contains 557.1 g of ethylene gl...
Questions

Mathematics, 22.09.2021 01:00




Mathematics, 22.09.2021 01:00

Mathematics, 22.09.2021 01:00


Mathematics, 22.09.2021 01:00

Health, 22.09.2021 01:00


Mathematics, 22.09.2021 01:00


Social Studies, 22.09.2021 01:00


English, 22.09.2021 01:00



History, 22.09.2021 01:00


Mathematics, 22.09.2021 01:00

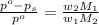
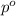 = vapor pressure of pure solvent (water) = 760 torr
= vapor pressure of pure solvent (water) = 760 torr 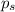 = vapor pressure of solution = ?
= vapor pressure of solution = ?
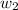 = mass of solute (ethylene glycol) = 557.1 g
= mass of solute (ethylene glycol) = 557.1 g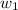 = mass of solvent (water) = 1000.0 g
= mass of solvent (water) = 1000.0 g
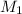 = molar mass of solvent (water) = 18.02 g/mole
= molar mass of solvent (water) = 18.02 g/mole
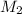 = molar mass of solute (ethylene glycol) = 62.07 g/mole
= molar mass of solute (ethylene glycol) = 62.07 g/mole