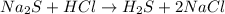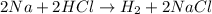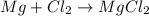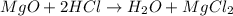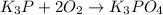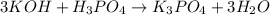Chemistry, 02.09.2019 20:30 makaylarae8781
Classify the following as acid-base reactions or oxidation-reduction reactions:
(a)na2s + hcl -> h2s + 2nacl
(b)2na + 2hcl -> h2 + 2nacl
(c)mg + cl2 = mgcl2
(d)mgo + 2hcl = h2o + mgcl2
(e)k3p+2o2 -> k3po4
(f)3koh +h3po4 -> k3po4 + 3h2o

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 22:00
Afamily is one another name for a group on the table of elements.
Answers: 1

Chemistry, 22.06.2019 13:30
Which of the following has wavelength longer than the wavelength of viable light? a) x rays b) gamma rays c) radios waves d) ultraviolet waves
Answers: 1

Chemistry, 22.06.2019 21:30
Which of the following changes will decrease the total amount of gaseous solute able to be dissolved in a liter of liquid water? (2 points) decreasing temperature decreasing pressure decreasing surface area decreasing solute concentration
Answers: 1

Chemistry, 22.06.2019 23:30
How many grams of ammonia would be produced by the decomposition of 16.93 mlof hydrazine? (the density of hydrazine is 1.021g/ml)
Answers: 3
You know the right answer?
Classify the following as acid-base reactions or oxidation-reduction reactions:
(a)na2s + hcl...
(a)na2s + hcl...
Questions


English, 19.09.2019 22:30


Mathematics, 19.09.2019 22:30

History, 19.09.2019 22:30







Mathematics, 19.09.2019 22:30

Biology, 19.09.2019 22:30



Mathematics, 19.09.2019 22:30

History, 19.09.2019 22:30

Mathematics, 19.09.2019 22:30