
Chemistry, 02.09.2019 21:10 lekaje2375
Identify the atoms that are oxidized and reduced, the change in the oxidation state for each, and oxidising and reducing agents in each of the following equations:
(a)mg + nicl2 -> mgcl2 + ni
(b)pcl3 + cl2 -> pcl5
(c)c2h4 + 3o2 -> 2co2 + 2h2o
(d)zn + h2so4 -> h2 + znso4
(e)2k2s2o3 + i2- > k2s4o6+2ki
(f)3cu + 8hno3 = 3cu(no3)2 + 4h2o + 2no

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 20:30
Which of the following pairs of elements belong to the same groupa. h and he b. li and bec. c and pb d. ga and ge
Answers: 1

Chemistry, 21.06.2019 22:30
Asolution contains 225 g of sodium chloride, nacl, dissolved in enough water to make a 0.25 l of solution. what is the molarity of the solution? a. 3.88 m, b. 1.03 m, c. 1.5 m, d. 15.5 m
Answers: 3


Chemistry, 22.06.2019 09:20
What will most likely happen when two bromine atoms bond together?
Answers: 3
You know the right answer?
Identify the atoms that are oxidized and reduced, the change in the oxidation state for each, and ox...
Questions


Mathematics, 13.05.2021 23:10


Mathematics, 13.05.2021 23:10



Computers and Technology, 13.05.2021 23:10





Biology, 13.05.2021 23:10

SAT, 13.05.2021 23:10



Mathematics, 13.05.2021 23:10

Mathematics, 13.05.2021 23:10



Social Studies, 13.05.2021 23:10

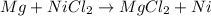
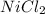 acts as oxidizing agent.
acts as oxidizing agent.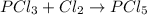
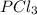 acts as reducing agent and
acts as reducing agent and 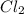 acts as oxidizing agent.
acts as oxidizing agent.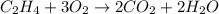
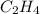 acts as reducing agent and
acts as reducing agent and 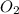 acts as oxidizing agent.
acts as oxidizing agent.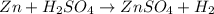
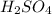 acts as oxidizing agent
acts as oxidizing agent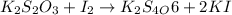
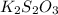 acts as reducing agent and
acts as reducing agent and 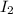 acts as oxidizing agent.
acts as oxidizing agent.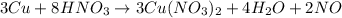
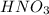 acts as oxidizing agent
acts as oxidizing agent

