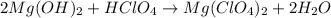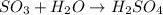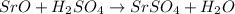Chemistry, 02.09.2019 21:10 jesusmojica25
Complete and balance the equation for the following acid-base neutralization reaction. if water is used as a solvent, write the reactants and products as aqueous ions. in some cases, there may be more than one correct answer, depending on the amount of reactants used.
(a)mg(oh)2 + hclo4?
(b)so3 + h2o? (assume an excess of water and that the product dissolves)
(c) sro + h2so4?

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 19:30
Motivation cannot be developed with practice; a person either possesses it or they do not.
Answers: 1

Chemistry, 22.06.2019 10:00
Americium-241 undergoes fission to produce three neutrons per fission event. if a neutron-absorbing material is mixed in with this sample so that the rate of neutron production drops down to 1.8 neutrons per fission event, which will be effective at achieving a critical mass? check all that apply. remove a deflective shield surrounding the sample. remove absorbent material mixed in with the sample. compress the sample of americium-241.
Answers: 1

Chemistry, 22.06.2019 16:00
Which factor is likely to impact the possible number of compounds ?
Answers: 1

Chemistry, 22.06.2019 23:00
What is the solubility-product constant of barium sulfate, baso4, if a saturated solution is 1.03 ´ 10-5 m?
Answers: 3
You know the right answer?
Complete and balance the equation for the following acid-base neutralization reaction. if water is u...
Questions



Social Studies, 17.03.2021 23:50

Mathematics, 17.03.2021 23:50


Mathematics, 17.03.2021 23:50

Mathematics, 17.03.2021 23:50


Mathematics, 17.03.2021 23:50

Mathematics, 17.03.2021 23:50


Biology, 17.03.2021 23:50


English, 17.03.2021 23:50

Mathematics, 17.03.2021 23:50




Computers and Technology, 17.03.2021 23:50

Mathematics, 17.03.2021 23:50

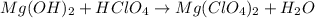
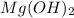 by 2 on reactant side and multiply
by 2 on reactant side and multiply 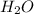 by 2 on product side.
by 2 on product side.