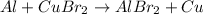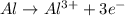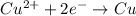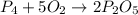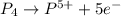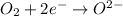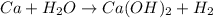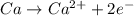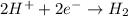Complete and balance the following oxidation-reduction reactions, which give the highest possible oxidation state for the
oxidizing atoms:
(a)al + f2?
(b)al + cubr2 ? (single displacement)
(c)p4 + o2?
(d)ca + h2o ? (products are a strong base and a diatomic gas)

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 06:00
If you burn 10 kilograms of wood in a fire (combustion) what is the weight of the products after the fire has finished burning the wood?
Answers: 3

Chemistry, 22.06.2019 06:10
Explain the relationship between forward and backward reactions in equilibrium, and predict how changing the amount of a reactant (creating a tension) will affect that relationship.
Answers: 1


Chemistry, 22.06.2019 06:20
If i can still dissolve more sugar into the solution at a certain temperature what would i call that solution
Answers: 3
You know the right answer?
Complete and balance the following oxidation-reduction reactions, which give the highest possible ox...
Questions






Mathematics, 05.03.2022 17:40


Mathematics, 05.03.2022 17:40

Chemistry, 05.03.2022 17:40

Mathematics, 05.03.2022 17:40





Spanish, 05.03.2022 17:40


Social Studies, 05.03.2022 17:40

Mathematics, 05.03.2022 17:40

Mathematics, 05.03.2022 17:40

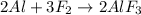
![Al\rightarrow Al^{3+]+3e^-](/tpl/images/0221/3679/62359.png)