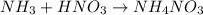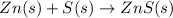Chemistry, 02.09.2019 21:30 Spencerg325
Write balanced chemical equation for the reactions used to prepare each of the following compounds from the given starting material(s). in some cases, additional reactants may be required.
(a) solid ammonium nitrate from gaseous molecule of nitrogen viaa two step process (first reduce the nitrogen to ammonia than neutrlize the ammonium in an appropriate acid)
(b)gaseous hydrogen bromide liquid molecular bromin via one step redox reaction
(c)gaseous h2s from solid zn and s via a two step process(first a redox reaction between the starting material then reaction of the product with a strong acid)

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:30
1. combine iron and copper (ii) sulfate solution. (hint: iron will form the iron (iii) ion) fe + cuso4 → 2. combine lead (ii) nitrate and potassium iodide solutions. pb(no3)2+ kl → 3. combine magnesium metal and hydrochloric acid solution. mg + hcl → 4. electrolysis (splitting) of water. h2o → 5. burning magnesium. mg + o2 →
Answers: 3

Chemistry, 22.06.2019 06:00
Calculate - analysis of compound composed of iron and oxygen yields 174.86 of fe and 75.14g of o. what is the empirical formula for this compound?
Answers: 3

Chemistry, 22.06.2019 16:30
Asample of freon gas has a volume of 2.23 liters, a pressure of 4.85 kpa, and a temperature of -1.36°c. calculate the volume at a pressure of 1.38 kpa and a temperature of 5.5°c. (show work)
Answers: 1

Chemistry, 23.06.2019 05:30
How many moles are in 1.26*10^24 particles in significant figures
Answers: 2
You know the right answer?
Write balanced chemical equation for the reactions used to prepare each of the following compounds f...
Questions

Social Studies, 31.01.2020 07:47

History, 31.01.2020 07:47

Health, 31.01.2020 07:47


Social Studies, 31.01.2020 07:47

Mathematics, 31.01.2020 07:47

Social Studies, 31.01.2020 07:47


Mathematics, 31.01.2020 07:47

Mathematics, 31.01.2020 07:48

Mathematics, 31.01.2020 07:48


Mathematics, 31.01.2020 07:48

History, 31.01.2020 07:48


Mathematics, 31.01.2020 07:48

Mathematics, 31.01.2020 07:48