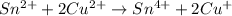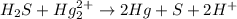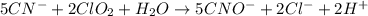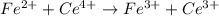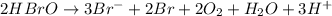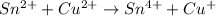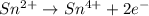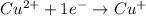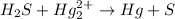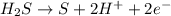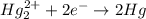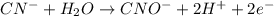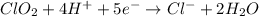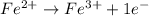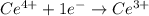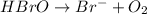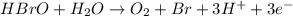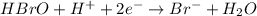Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 03:40
Chemical kinetics what was the rate of reaction in trial 3? choose the closest answer.
Answers: 3


Chemistry, 22.06.2019 20:30
Which of the following is not true about the atomic model of substances?
Answers: 1

Chemistry, 23.06.2019 02:00
Scientists are often interested in knowing the molar heat of combustion – the heat released during the combustion of one mole of a substance. use the periodic table to find molar masses. how many moles of ethanol are present in the sample?
Answers: 2
You know the right answer?
Balance each of the following equations according to the half- reaction method:
(a) sn2+ + cu...
(a) sn2+ + cu...
Questions

Physics, 20.10.2020 21:01


Mathematics, 20.10.2020 21:01

History, 20.10.2020 21:01

Mathematics, 20.10.2020 21:01




English, 20.10.2020 21:01





English, 20.10.2020 21:01

History, 20.10.2020 21:01

Mathematics, 20.10.2020 21:01


Mathematics, 20.10.2020 21:01

Mathematics, 20.10.2020 21:01

Social Studies, 20.10.2020 21:01