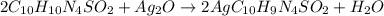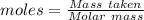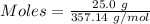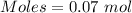Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 14:00
Calculate the energy required to ionize a hydrogen atom to an excited state where the electron is initially in the n = 5 energy level. report your answer in kilojoules
Answers: 1

Chemistry, 22.06.2019 19:30
What is the area in square meters of 448 g ai foil that has a thickness of 23921 nm? the density is 2.70 g/cm
Answers: 3

Chemistry, 22.06.2019 21:00
Need what is special about water as a compound? how does water regulate climate? what drives water evaporation? why is the water vapor fresh water when it rises from the ocean? why might freshwater in the form of snow take longer to enter the water cycle again than liquid precipitation? what is an aquifer? what role do people play in the water cycle? plz just answer as many as you can ! thx if you !
Answers: 1

Chemistry, 23.06.2019 05:00
How many atomic mass units are equal to 1.672×10−24 g of protons?
Answers: 3
You know the right answer?
What mass of silver oxide, ag2o is required to produce 25.0 g of silver sulfadiazine, agc10h9n4so2,...
Questions

Mathematics, 25.02.2021 19:20

English, 25.02.2021 19:20




Physics, 25.02.2021 19:20

English, 25.02.2021 19:20








Mathematics, 25.02.2021 19:20


Business, 25.02.2021 19:20

Mathematics, 25.02.2021 19:20

Mathematics, 25.02.2021 19:20