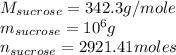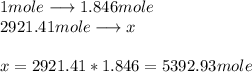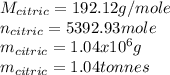Chemistry, 02.09.2019 23:20 andrwisawesome0
Citric acid, c6h8o7, a component of jams, jells and fruity soft drinks, is prepared industrially via a fermentation of sucrose by the moid aspergillus niger. the equation representing this raction id :
c12h22o11 + h2o + 3o2 ? 2c6h8o7 +h2o
what mass of nitric acid is produced from exactly 1 metric on(1000 x 103 kg) of sucrose if the yield is 92.30%?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 09:00
An excess of lithium oxide undergoes a synthesis reaction with water to produce lithium hydroxide li2o+h2o→2lioh if 1.05 g of water reacted, what is the theoretical yield of lithium hydroxide? a) 5.83 x 10–2 g lioh b) 1.17 x 10–1 g lioh c) 2.79 x 100 g lioh d) 1.40 x 100 g lioh
Answers: 1


Chemistry, 22.06.2019 15:00
20 pts ‼️ an unmanned spacecraft travels to mars. mars has a lower strength of gravity than earth. where in the image is the spacecraft’s weight the greatest?
Answers: 1

You know the right answer?
Citric acid, c6h8o7, a component of jams, jells and fruity soft drinks, is prepared industrially via...
Questions


Mathematics, 04.10.2021 09:40


English, 04.10.2021 09:40

Mathematics, 04.10.2021 09:50

Mathematics, 04.10.2021 09:50

Mathematics, 04.10.2021 09:50



Biology, 04.10.2021 09:50


History, 04.10.2021 09:50

Health, 04.10.2021 09:50



Mathematics, 04.10.2021 09:50


Chemistry, 04.10.2021 09:50

Mathematics, 04.10.2021 09:50