Chemistry, 03.09.2019 00:10 thedragontale1020
Calculate for the following electrochemical cell at 25°c, pt h2(g) (1.0 atm) h (0.010 m || ag (0.020 m) ag if e (h) - +0.000 v and e(ag)-0.799 v. a. +0.817 v b. +0.799 v c. +0.911 v d. +0.275 v e. +1.01 v ,

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 16:30
Acetic acid, hc2h3o2, dissolves and completely dissociates in water and a solvation sphere of water molecules forms around the ions. this solute-solvent interaction
Answers: 1

Chemistry, 22.06.2019 21:30
How many oxygen atoms are there in 3.15 moles of hcl manganese (iv) oxide, mno2
Answers: 2

Chemistry, 23.06.2019 00:00
How do you determine the percent yield of a chemical reaction
Answers: 1

Chemistry, 23.06.2019 19:30
There was a water with a boat and a rock in a boat. will the level of the water stay the same or higher or lower if u remove the rock from the boat and put it under water? ?
Answers: 3
You know the right answer?
Calculate for the following electrochemical cell at 25°c, pt h2(g) (1.0 atm) h (0.010 m || ag (0.020...
Questions

Computers and Technology, 28.01.2020 16:04





History, 28.01.2020 16:04


World Languages, 28.01.2020 16:04


Mathematics, 28.01.2020 16:04






Advanced Placement (AP), 28.01.2020 16:04

English, 28.01.2020 16:04

Mathematics, 28.01.2020 16:04

Mathematics, 28.01.2020 16:04

Mathematics, 28.01.2020 16:04

![E^0_{[H^{+}/H_2]}=+0.00V](/tpl/images/0221/4856/30abe.png)
![E^0_{[Ag^{+}/Ag]}=+0.799V](/tpl/images/0221/4856/2c999.png)
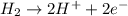
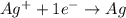
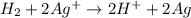
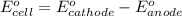
![E^o_{cell}=E^o_{[Ag^{+}/Ag]}-E^o_{[H^{+}/H_2]}](/tpl/images/0221/4856/e75b5.png)