
Chemistry, 03.09.2019 00:20 mahogany1956
Ammonia (nh3) reacts with oxygen to form nitric oxide (no) and water vapor: 4nh3 + 502 4no + 6h2o b) when 20.0 g nh3 and 50.0 g o2 are allowed to react, which is the limiting reagent? which reactant is left over and how much left over at the end of reaction? ans: 2.94 g c) what is the theoretical yield of no if 20.0 g nh3 and 50.0 g o2 are allowed to react? ans: 35.29 g d) what is the theoretical yield of h20 if 20.0 g nh3 and 50.0 g o2 are allowed to react? ans: 31.74 g

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:30
If you want to create an electrical current, which situation would produce a solution capable of this
Answers: 3

Chemistry, 21.06.2019 23:00
An electrons position cannot be known precisely only it's probability of being in a certain location can be known
Answers: 1

Chemistry, 22.06.2019 18:20
Which reason best explains why metals are malleable? a)because they have delocalized electrons b)because they have localized electrons c)because they have ionic bonds d)because they have rigid bonds
Answers: 2

Chemistry, 22.06.2019 19:50
Which sentence from holes contains an implied personality trait? stanley and his parents had tried to pretend that he was just going away to camp for a while, just like rich kids do. he'd just been in the wrong place at the wrong time. stanley felt somewhat dazed as the guard unlocked his handcuffs and led him off the bus. stanley nodded to show he understood
Answers: 3
You know the right answer?
Ammonia (nh3) reacts with oxygen to form nitric oxide (no) and water vapor: 4nh3 + 502 4no + 6h2o b...
Questions


English, 24.10.2021 23:30

Biology, 24.10.2021 23:30

English, 24.10.2021 23:30

Mathematics, 24.10.2021 23:30

English, 24.10.2021 23:30

English, 24.10.2021 23:30

Biology, 24.10.2021 23:30

Mathematics, 24.10.2021 23:30


Law, 24.10.2021 23:40



Social Studies, 24.10.2021 23:40



Mathematics, 24.10.2021 23:40

Mathematics, 24.10.2021 23:40


 is 35.29 g.
is 35.29 g. is 31.74 g.
is 31.74 g.





 of oxygen
of oxygen of
of 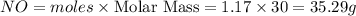
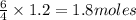 of
of 


