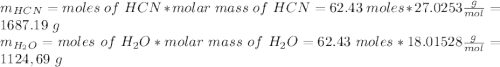Hydrogen cyanide is produced industrially from the reaction of gaseous ammonia, oxygen, and methane: 2nh3 1g2 1 3o2 1g2 1 2ch4 1g2 h 2hcn1g2 1 6h2o1g2 if 5.00 3 103 kg each of nh3, o2, and ch4 are reacted, what mass of hcn and of h2o will be produced, assuming 100% yield?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 02:50
The conventional equilibrium constant expression (kc) for the system below is: 2icl(s) ⇄ i2(s) + cl2(g) [cl2] ([i2] + [cl2])/2[icl] [i2][cl2]/[icl]2 none of the listed answers are correct [i2][cl2]/2[icl]
Answers: 2

Chemistry, 22.06.2019 19:10
How does the atmosphere to make earth livable? check all that apply. causes the seasons contains oxygen provides warmth creates important nutrients blocks harmful energy from the sun plz like !
Answers: 2

Chemistry, 23.06.2019 19:00
The mass of a substance that has a density of 10.0g/ml and a volume of 6.0ml is
Answers: 1

Chemistry, 23.06.2019 23:30
How does a sample of hydrogen at 10°c compared to a sample of hydrogen at 350 kelvin?
Answers: 1
You know the right answer?
Hydrogen cyanide is produced industrially from the reaction of gaseous ammonia, oxygen, and methane:...
Questions





Spanish, 19.04.2020 02:26


Mathematics, 19.04.2020 02:26

History, 19.04.2020 02:26



Mathematics, 19.04.2020 02:26

History, 19.04.2020 02:27

Mathematics, 19.04.2020 02:27


Mathematics, 19.04.2020 02:27

Mathematics, 19.04.2020 02:27

Mathematics, 19.04.2020 02:27

Mathematics, 19.04.2020 02:27


History, 19.04.2020 02:28

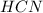 and 1124,69 g of
and 1124,69 g of 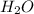
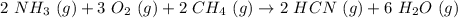
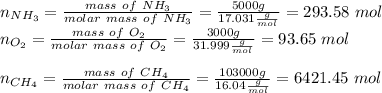
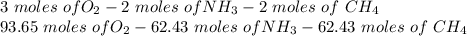
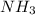 and
and 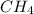 to consume the oxygen. In case there wasn´t enough of one of the other reactants, that one would be the limiting one.
to consume the oxygen. In case there wasn´t enough of one of the other reactants, that one would be the limiting one.