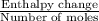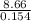In a coffee-cup calorimeter, 140.0 ml of 1.1 m and 140.0 ml of 1.1 m are mixed. both solutions were originally at 25.0°c. after the reaction, the final temperature is 32.4°c. assuming that all the solutions have a density of 1.0 and a specific heat capacity of 4.18 j/°c ⋅ g, calculate the enthalpy change for the neutralization of by . assume that no heat is lost to the surroundings or to the calorimeter. enthalpy change = kj/mol

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 05:30
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 1


Chemistry, 22.06.2019 13:10
What type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? view available hint(s) what type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? salt bridge disulfide bridge hydrogen bond hydrophobic interaction
Answers: 1
You know the right answer?
In a coffee-cup calorimeter, 140.0 ml of 1.1 m and 140.0 ml of 1.1 m are mixed. both solutions were...
Questions

Chemistry, 26.03.2020 20:02





Mathematics, 26.03.2020 20:02


Mathematics, 26.03.2020 20:02





Computers and Technology, 26.03.2020 20:02



Mathematics, 26.03.2020 20:02