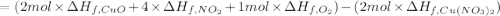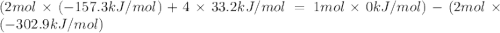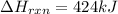The decomposition of copper(ii) nitrate on heating is endothermic reaction. 2cu(no3)2(s) → 2c10(s) + 4no2(g) + o2(g) calculate the enthalpy change for this reaction using the following enthalpy changes of formation. ah! [cu(no3)2) = -302.9 kj mol? ah, (cuo) = -157.3 kj mol? . ah[no2) = +33.2 kj mol.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 15:30
Determine the empirical formula of a compound containing 40.6 grams of carbon, 5.1 grams of hydrogen, and 54.2 grams of oxygen. in an experiment, the molar mass of the compound was determined to be 118.084 g/mol. what is the molecular formula of the compound? for both questions, show your work or explain how you determined the formulas by giving specific values used in calculations.
Answers: 3

Chemistry, 22.06.2019 03:10
Agas diffuses 1/7 times faster than hydrogen gas (h2). what is the molar mass of the gas? 100.10 g/mol 98.78 g/mol 86.68 g/mol 79.98 g/mol
Answers: 3

Chemistry, 22.06.2019 21:50
Liquid from a brewery fermentation contains 10% ethanol and 90% water. part of the fermentation product (50,000 kg/h) is pumped to a distillation column on the factory site. under current operating conditions, a distillate of 45% ethanol and 55% water is produced from the top of the column at a rate of one-tenth that of the feed. what is the composition of the waste "bottoms" from the still?
Answers: 2

Chemistry, 22.06.2019 23:00
What is the oxidation state of an individual bromine atom in nabro3?
Answers: 2
You know the right answer?
The decomposition of copper(ii) nitrate on heating is endothermic reaction. 2cu(no3)2(s) → 2c10(s) +...
Questions

English, 09.04.2020 22:17

Mathematics, 09.04.2020 22:17


Mathematics, 09.04.2020 22:17



English, 09.04.2020 22:17


Mathematics, 09.04.2020 22:17

Mathematics, 09.04.2020 22:17



English, 09.04.2020 22:17


Mathematics, 09.04.2020 22:17


Mathematics, 09.04.2020 22:17


History, 09.04.2020 22:17

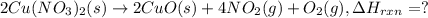
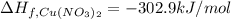
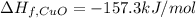
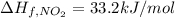
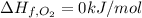 (standard state)
(standard state)![\Delta H_{rxn}=\sum [\Delta H_f(product)]-\sum [\Delta H_f(reactant)]](/tpl/images/0221/5790/84aad.png)
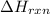 =
=