
Chemistry, 03.09.2019 03:30 tilly40oooo
The half-life for the second-order decomposition of hi is 15.4 s when the initial concentration of hi is 0.67 m. what is the rate constant for this reaction? a) 1.0 * 10-2 m-15-1 b) 4.5 * 10-2 m-15-1 c) 9.7* 10-2 m-15-1 od) 2.2 * 10-2 m-15-1

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 00:30
This is a characteristic of the elements in the periodic table that shows a pattern. it may increase or decrease across or down the table.
Answers: 1

Chemistry, 22.06.2019 04:30
What are the primary responsibilities of a chemical engineer involved in "r& d"? develop large scale manufacturing operations discover new products and processes training of new chemists determine products needed by consumers
Answers: 2

Chemistry, 22.06.2019 04:30
Using the periodic table, complete the table to describe each atom. type in your answers
Answers: 3

You know the right answer?
The half-life for the second-order decomposition of hi is 15.4 s when the initial concentration of h...
Questions



Mathematics, 26.04.2021 21:20


English, 26.04.2021 21:20

World Languages, 26.04.2021 21:20

English, 26.04.2021 21:20


Spanish, 26.04.2021 21:20



Business, 26.04.2021 21:20

Mathematics, 26.04.2021 21:20

Biology, 26.04.2021 21:20

Social Studies, 26.04.2021 21:20





English, 26.04.2021 21:20

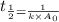
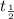 = half life = 15.4 s
= half life = 15.4 s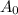 = initial concentration = 0.67 M
= initial concentration = 0.67 M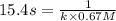
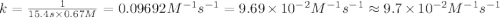
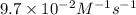 is the rate constant for this reaction.
is the rate constant for this reaction.

