
Which of the following states is true? (a) q does not change with temperature. (b) q is the same as keq when a reaction is at equilibrium. (c) key does not change with temperature, whereas q is temperature dependent. (d) k does not depend on the concentration or partial pressures of reaction components. (e) q does not depend on the concentrations or partial pressures of reaction components.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 18:30
Two people each hold the end of a rope and create waves by moving their arms up and down. this wave is best classified as a transverse wave because a) both the rope particles and the wave are moving in the same direction. b) the wave is moving up and down as the particles of the rope move horizontally. c) the wave is moving horizontally as the particles of the rope move up and down. eliminate d) the wave is moving in a parallel direction with the motion of the person's arms.
Answers: 3

Chemistry, 22.06.2019 21:00
Once similarity and one difference between a mixture of elements and a mixture of compounds
Answers: 3

Chemistry, 23.06.2019 00:30
What would be the original temperature of a gas that has a volume of 2.0 l and a pressure of 2.0 atm and an unknown temperature that the volume increased to 3.5 l in its pressure decreased to 1.0 atm if the final temperature is measured to be 11°c
Answers: 1

You know the right answer?
Which of the following states is true? (a) q does not change with temperature. (b) q is the same as...
Questions

Physics, 29.02.2020 19:27

Physics, 29.02.2020 19:27


Mathematics, 29.02.2020 19:29

Mathematics, 29.02.2020 19:29

Physics, 29.02.2020 19:29

Mathematics, 29.02.2020 19:31

Mathematics, 29.02.2020 19:32



Biology, 29.02.2020 19:37

Mathematics, 29.02.2020 19:38


Mathematics, 29.02.2020 19:38

Health, 29.02.2020 19:38

Biology, 29.02.2020 19:38

Mathematics, 29.02.2020 19:39



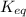 is the equilibrium constant and it is also equal to the concentration of products divided by the concentration of reactants. Also,
is the equilibrium constant and it is also equal to the concentration of products divided by the concentration of reactants. Also, 

