
Chemistry, 03.09.2019 18:30 isabellemaine
Tungsten trioxide (wo3) has a rich yellow color and is often used as a pigment in ceramics and paints. in order to test a ceramic vase for its wo3 content, a 10.29 g sample of the vase was ground and reduced with pb(hg) to convert any wo3 to w3 . the resulting w3 was transferred to 500.0 ml of 1.00 m hcl. a 100.00 ml aliquot of the hcl solution required 11.43 ml of 0.09713 m potassium permanganate (kmno4) to reach the purple endpoint. a blank required 0.25 ml. balance the reaction below and determine the percent wo3 in the ceramic sample.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 07:20
Why does his teacher ask him to balance the equation by including the correct coefficient
Answers: 1


Chemistry, 22.06.2019 17:00
What is the approximate vapor pressure when the gas condenses at 70 degrees celsius
Answers: 2

Chemistry, 23.06.2019 02:30
Apound is approximately 0.45 kilogram. a persons weighs 87 kilograms. what is the persons’s weight, in pounds, when expressed to the correct number of significant figures
Answers: 1
You know the right answer?
Tungsten trioxide (wo3) has a rich yellow color and is often used as a pigment in ceramics and paint...
Questions


Mathematics, 18.10.2020 06:01




History, 18.10.2020 06:01


Mathematics, 18.10.2020 06:01

Mathematics, 18.10.2020 06:01

History, 18.10.2020 06:01

Chemistry, 18.10.2020 06:01


Mathematics, 18.10.2020 06:01



Social Studies, 18.10.2020 06:01

Mathematics, 18.10.2020 06:01

Spanish, 18.10.2020 06:01


Mathematics, 18.10.2020 06:01

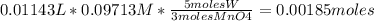
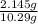 * 100% = 20.84%
* 100% = 20.84%

