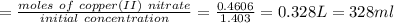Chemistry, 03.09.2019 18:30 dabicvietboi
Calculate the following quantity: volume of 1.403 m copper(ii) nitrate that must be diluted with water to prepare 575.2 ml of a 0.8012 m solution.

Answers: 1
Another question on Chemistry


Chemistry, 21.06.2019 17:30
What is the formula for the molecular compound nitrogen monoxide
Answers: 1

Chemistry, 22.06.2019 05:50
According to coulomb's law, how would the electrical force between particles change if the product of their electrical charge increased?
Answers: 1

Chemistry, 22.06.2019 08:30
Agroup of students is studying convection current. they fill two identical balloons with the same amount of helium. one balloon is placed in a freezer and the other is in an area with warm air. after 10 minutes, the balloon are released from a height of 1 meter. which of the following to the students most likely observe? a) the warm balloon expands and rises. the cold balloon shrinks and sinks b) the balloon both rise. the cold balloon is larger than the warm balloon c) the cold balloon expands and rises. the warm balloon shrinks and sinks d) the balloon rise at the same rate. both balloons are the same size
Answers: 1
You know the right answer?
Calculate the following quantity: volume of 1.403 m copper(ii) nitrate that must be diluted with wa...
Questions




SAT, 24.12.2021 05:40






Health, 24.12.2021 05:50

SAT, 24.12.2021 05:50

SAT, 24.12.2021 05:50

Social Studies, 24.12.2021 05:50


SAT, 24.12.2021 05:50


SAT, 24.12.2021 05:50

SAT, 24.12.2021 05:50

SAT, 24.12.2021 05:50

Spanish, 24.12.2021 05:50