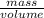Chemistry, 03.09.2019 19:30 qwertylol12345
The density of copper decreases as temperature increases (as does the density of most substances). which change occurs in a sample of copper when it is warmed from room temperature to 95 °c?
(a) the sample will become lighter.
(b) the sample will become heavier.
(c) the sample will expand.
(d) the sample will contract.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 07:00
In the cathode ray tube experiment, j. j. thomson passed an electric current through different gases inside a cathode ray tube in the presence of an electric field. in which two ways did this experiment change scientists’ understanding of the atom?
Answers: 2

Chemistry, 22.06.2019 22:30
Molecular iodine, i2(g), dissociates into iodine atoms at 625 k with a first-order rate constant of 0.271 s−1. part a part complete what is the half-life for this reaction?
Answers: 3

Chemistry, 23.06.2019 05:10
Will mark as brainliest ! how many grams of iron metal do you expect to be produced when 245 grams of an 80.5 percent by mass iron (ii) nitrate solution react with excess aluminum metal? show all work needed to solve this problem!
Answers: 1

Chemistry, 23.06.2019 07:00
Why do the strengths of london (dispersion) forces generally increase with increasing molecular size? choose one: a. heavier atoms have stronger attractions for each other than lighter atoms. b. dispersion forces are all equal in magnitude; there is no size dependence. c. dispersion forces arise from the attraction between the nuclei of atoms, and larger molecules have larger nuclei. d. dispersion forces arise from dipoles caused by the electron distribution being distorted. larger molecules have more electrons and, therefore, more distortions and a bigger force. e. dispersion forces depend on distance. larger molecules are farther apart and so the forces are smaller.
Answers: 2
You know the right answer?
The density of copper decreases as temperature increases (as does the density of most substances). w...
Questions


English, 31.05.2021 22:10

Arts, 31.05.2021 22:10

Biology, 31.05.2021 22:10


Biology, 31.05.2021 22:10



Arts, 31.05.2021 22:10

Health, 31.05.2021 22:10

Mathematics, 31.05.2021 22:10



Mathematics, 31.05.2021 22:10


Business, 31.05.2021 22:10

Mathematics, 31.05.2021 22:10


Mathematics, 31.05.2021 22:10

English, 31.05.2021 22:10