Chemistry, 03.09.2019 20:30 montoyaricardo3550
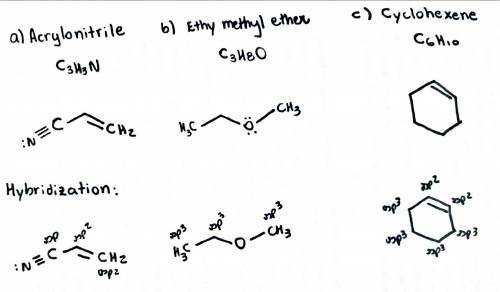
Draw the structures for the following molecules (show all lone pairs): a) acrylonitrile, c3h3n, which contains a carbon-carbon double bond and a carbon-nitrogen triple bond. b) ethyl methyl ether, c3h8o, which contains an oxygen atom bonded to two carbons. c) cyclohexene, c6h10, which contains a ring of six atoms and one carbon-carbon double bond. d) determine the hybridization, the shape, the bond angle and the polarity of each of the structures.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 12:50
Which phrase best describes the rock's texture? 1.jagged grains 2.coarse grains 3.rounded grains 4.non-banded grains
Answers: 1

Chemistry, 22.06.2019 16:00
What rule is used to determine how many covalent bonds an element can form? a. the number of covalent bonds is equal to six c the number of covalent bonds is equal to five minus the group number plus the group number b. the number of covalent bonds is equal to eight d. none of the above minus the group number select the best answer from the choices provided
Answers: 2

Chemistry, 22.06.2019 17:00
Reduction is a reaction which results in a in electrons and a in positive charge of the atom or ion 1) a- loss 1) b- gain 2) a-increase 2) b-decrease
Answers: 1

You know the right answer?
Draw the structures for the following molecules (show all lone pairs): a) acrylonitrile, c3h3n, whic...
Questions

Physics, 26.01.2021 21:30






Computers and Technology, 26.01.2021 21:30



Mathematics, 26.01.2021 21:30


Social Studies, 26.01.2021 21:30

Mathematics, 26.01.2021 21:30

Social Studies, 26.01.2021 21:30

English, 26.01.2021 21:30

Mathematics, 26.01.2021 21:30

Arts, 26.01.2021 21:30

Geography, 26.01.2021 21:30

Mathematics, 26.01.2021 21:30