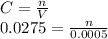Chemistry, 03.09.2019 21:30 champions2k19
A25.0-ml sample containing cu2+ gave an instrument signal of 25.2 units (corrected for a blank). when exactly 0.500 ml of 0.0275 m cu(no3)2 was added to the solution, the signal increased to 45.1 units. calculate the molar concentration of cu2+ assuming that the signal was directly proportional to the analyte concentration. skoog, douglas a.. principles of instrumental analysis (p. 20). brooks cole. kindle edition.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 02:30
The is a particle with one unit of positive charge a. proton b. positron c. electron d. nucleus awnser quick it is a important science test!
Answers: 2



Chemistry, 22.06.2019 14:00
Which of the following is true about a carbonated soft drink? . the carbon dioxide is the solvent, and water is the solute.. the water is the solution, and carbon dioxide is the solvent.. the carbon dioxide is the solution, and the water is the solvent.. the water is the solvent, and the carbon dioxide is the solute.. .
Answers: 1
You know the right answer?
A25.0-ml sample containing cu2+ gave an instrument signal of 25.2 units (corrected for a blank). whe...
Questions


Mathematics, 26.05.2021 21:40


Mathematics, 26.05.2021 21:40



Mathematics, 26.05.2021 21:40



Mathematics, 26.05.2021 21:40


Mathematics, 26.05.2021 21:40


Mathematics, 26.05.2021 21:40


Biology, 26.05.2021 21:40

Mathematics, 26.05.2021 21:40

Mathematics, 26.05.2021 21:40

English, 26.05.2021 21:40

Mathematics, 26.05.2021 21:40