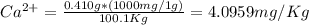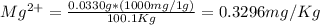Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 22:00
Which describes interactions between substances and stomata during photosynthesis? check all that apply. oxygen enters stomata. oxygen is released through stomata. carbon dioxide enters stomata. carbon dioxide is released through stomata. hydrogen enters stomata. hydrogen is released through stomata.
Answers: 1

Chemistry, 22.06.2019 02:30
Asa choose the correct set of reaction coefficients to properly balance the following chemical equation according to the law of conservation of mass: __s8 + __o2 ==> __so2 1, 1, 8 1, 8, 1 1, 8, 8 8, 1, 1
Answers: 1

Chemistry, 22.06.2019 05:00
What forms when chemical reactions combine pollution with sunlight?
Answers: 1

Chemistry, 22.06.2019 18:50
Asample of tin (ii) chloride has a mass of 0.49 g. after heating, it has a mass of 0.41 g. what is the percent by mass of water in the hydrate? %
Answers: 1
You know the right answer?
Wastewater from a cement factory contains 0.410 g of ca2+ ion and 0.0330 g of mg2+ ion per 100.0 l o...
Questions

Mathematics, 04.08.2019 14:00



History, 04.08.2019 14:00

History, 04.08.2019 14:00


Mathematics, 04.08.2019 14:00

Mathematics, 04.08.2019 14:00



Chemistry, 04.08.2019 14:00




English, 04.08.2019 14:00

Mathematics, 04.08.2019 14:00

English, 04.08.2019 14:00


Social Studies, 04.08.2019 14:00

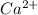 = 4.0959 ppm
= 4.0959 ppm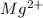 = 0.3296 ppm
= 0.3296 ppm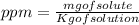
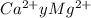
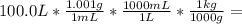 = 100.1 kg
= 100.1 kg