Chemistry, 04.09.2019 00:10 ashley5196
Sulfuric acid (h2so4) is prepared commercially from elemental sulfur using the contact process. in a typical sequence of reactions, the sulfur is first burned: s + o2 → so2 , then it is converted to so3 using a catalyst: 2 so2 + o2 → 2 so3 . the resulting so3 is reacted with water to produce the desired product: so3 + h2o → h2so4 . how much sulfuric acid could be prepared from 83 moles of sulfur? answer in units of g.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 17:30
Aspirin has a density of 1.40 g/cm3 what is the volume in cubic centimeters of a tablet weighing 320 mg ?
Answers: 1

Chemistry, 22.06.2019 10:00
The reactions shown here can be combined to make the overall reaction c(s) + h2o(g) ⇌ co(g) + h2(g) by reversing some and/or dividing all the coefficients by a number. a. c(s) + o2(g) → co2(g) k=1.363×10^69 b. 2 h2(g) + o2(g) → 2 h2o(g) k=1.389×10^80 c. 2co(g) + o2 (g) → 2 co2(g) k=1.477×10^90
Answers: 1

Chemistry, 22.06.2019 10:30
When the speed of the bottle is 2 m/s, the average maximum height of the beanbag is m.
Answers: 2

Chemistry, 22.06.2019 15:30
Count the number of each type of atom in the equation below, and then balance the equation. write in the numbers of atoms and coefficients. add a 1 if there should be no coefficient. cs2(l) + o2(g) → co2(g) + so2(g) c [ ] s [ ] o > c [ ] s [ ] o [ ] cs2(l) + [ ] o2(g) > [ ] co2(g) + [ ] so2(g)
Answers: 3
You know the right answer?
Sulfuric acid (h2so4) is prepared commercially from elemental sulfur using the contact process. in a...
Questions

Health, 20.08.2019 03:10




Health, 20.08.2019 03:10















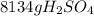
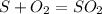
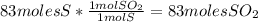
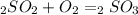
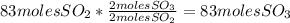
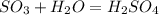
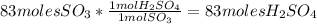
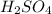 :
: