
Chemistry, 04.09.2019 02:20 jeanetteelliotp9ru6m
Consider two gases, a and b, each in a 1.0-l container with both gases at the same temperature and pressure. the mass of gas a in the container is 0.34 g and the mass of gas b in the container is 0.48 g.
a. which gas sample has the most molecules present? b. which gas sample has the largest average kinetic energy? c. which gas sample has the fastest average velocity? d. how can the pressure in the 2 containers be equal to each other since the larger gas b molecules collide with the container walls more forcefully?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 05:30
What royal scientist used the 29th day of frozen vapor to encounter elements for mastering new culinary creations?
Answers: 1

Chemistry, 22.06.2019 06:00
An atom of sodium-23 (atomic number = 11) has a positive charge of +1. give this information, how many electrons does it have? how many proteins and neutrons does this atom have
Answers: 2

Chemistry, 22.06.2019 09:00
Ineed to find the answer of this question because i dont understand it
Answers: 1

Chemistry, 22.06.2019 11:00
When hydrochloric acid reacts with potassium hydroxide solution, the following reaction occurs. hcl (aq) + koh (aq) h2o (l) + kcl (aq) the reaction gives off heat energy, so it is an reaction.
Answers: 1
You know the right answer?
Consider two gases, a and b, each in a 1.0-l container with both gases at the same temperature and p...
Questions


Physics, 06.10.2021 14:20

Chemistry, 06.10.2021 14:20

Mathematics, 06.10.2021 14:20


English, 06.10.2021 14:20


English, 06.10.2021 14:20

Mathematics, 06.10.2021 14:20

Mathematics, 06.10.2021 14:20

Mathematics, 06.10.2021 14:20

Biology, 06.10.2021 14:20

English, 06.10.2021 14:20

Mathematics, 06.10.2021 14:20


Chemistry, 06.10.2021 14:20

SAT, 06.10.2021 14:20

Physics, 06.10.2021 14:20


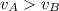 D) compensation between force and velocity.
D) compensation between force and velocity.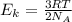 , where R is the ideal gas constant, T is the temperature and
, where R is the ideal gas constant, T is the temperature and 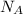 is the Avogadro´s number. All this values are the same in both gases thus they have the same kinetic energy.
is the Avogadro´s number. All this values are the same in both gases thus they have the same kinetic energy.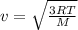 , where M is the molecular mass, if we want to know wich gas has fastest velocity is enough to know the rate between two velocities.
, where M is the molecular mass, if we want to know wich gas has fastest velocity is enough to know the rate between two velocities.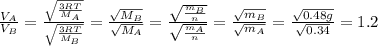 , due to rate is greater than 1
, due to rate is greater than 1 

