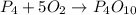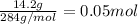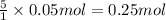Chemistry, 04.09.2019 05:20 vannybelly83
Given the unbalanced reaction: p₄+ o₂  p₄o₁₀ how many moles of o₂ are needed to produce 14.2 grams of p₄o₁₀?
p₄o₁₀ how many moles of o₂ are needed to produce 14.2 grams of p₄o₁₀?
a. 0.125 mol
b. 0.0620 mol
c. 0.500 mol
d. 0.0500 mol
e. 0.250 mol

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 05:30
Arecipe calls for 1.2 cups of oil. how many liters of oil is this?
Answers: 2

Chemistry, 22.06.2019 07:00
At 450 mm hg a gas has a volume of 760 l, what is its volume at standard pressure
Answers: 2

Chemistry, 22.06.2019 18:10
Consider the following reaction at equilibrium: c(s)+h2o(g)⇌co(g)+h2(g) predict whether the reaction will shift left, shift right, or remain unchanged upon each of the following disturbances. a) c is added to the reaction mixture. b) h2ois condensed and removed from the reaction mixture c) co is added to the reaction mixture d) h2 is removed from the reaction mixture.
Answers: 3

Chemistry, 22.06.2019 23:30
To find the work done, the force exerted and distance moved are multiplied. a couch is moved twice before you are happy with its placement. the same force was used to move the couch both times. if more work is done the first time it is moved, what do you know about the distance it was moved? a) when more work was done, the couch was moved the same distance. b) when more work was done, the couch was moved less. c) when more work was done, the couch was moved further. d) when more work was done, the couch wasn't moved at all.
Answers: 1
You know the right answer?
Given the unbalanced reaction: p₄+ o₂ [tex]\rightarrow[/tex] p₄o₁₀ how many moles of o₂ are needed...
Questions

Mathematics, 27.10.2019 18:43


Mathematics, 27.10.2019 18:43

Mathematics, 27.10.2019 18:43

Mathematics, 27.10.2019 18:43

Biology, 27.10.2019 18:43

Mathematics, 27.10.2019 18:43

Mathematics, 27.10.2019 18:43



Chemistry, 27.10.2019 18:43






Mathematics, 27.10.2019 18:43



Mathematics, 27.10.2019 18:43