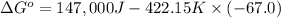Chemistry, 04.09.2019 17:10 AkramMasoud
Calculate the δg for the following system. then state if the system is spontaneous or not spontaneous. δh = 147 kj δs = -67.0 j/k t = 149 °c

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 18:50
Suppose you got a low yield of benzoin from your benzoin condensation reaction and thus only have 0.300 g of benzoin to use as the starting material for this reaction. how much concentrated nitric acid should you add? (concentrated nitric acid is 15.8 m). write your answer in the form x.xx ml
Answers: 1

Chemistry, 22.06.2019 06:30
Summarize possible ways in which phases of matter could combine to form a solution.
Answers: 2

Chemistry, 22.06.2019 19:00
How does a catalyst increase the speed of a reaction? a. the catalyst eliminates the activated complex stage, allowing products to form immediately. b. the catalyst lowers the energy level of the reactants, making it easier for them to react. c. the catalyst makes it easier for the activated complex to form, lowering the activation energy. d. the catalyst raises the energy level of the products, making the reaction finish sooner. reset next
Answers: 1

Chemistry, 22.06.2019 23:00
Which organism develops breathing organism develops breathing organs from pharyngeal arches? shark, spider, sea star, sea horse
Answers: 2
You know the right answer?
Calculate the δg for the following system. then state if the system is spontaneous or not spontaneou...
Questions

Spanish, 12.11.2020 07:10

Arts, 12.11.2020 07:10

History, 12.11.2020 07:10



Mathematics, 12.11.2020 07:10

Mathematics, 12.11.2020 07:10



Arts, 12.11.2020 07:10

Mathematics, 12.11.2020 07:10

English, 12.11.2020 07:10



Mathematics, 12.11.2020 07:10