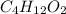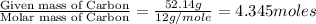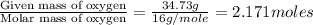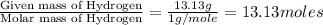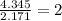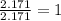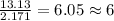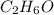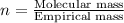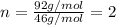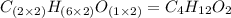Chemistry, 05.09.2019 01:10 fernandaretanaoxwln0
Determine the empirical formula for a compound that contains c, h and o. it contains 52.14% c and 34.73% o by mass. what is the molecular formula if the molar mass is 92 g/mol?

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 14:30
Find the mass in grams of hydrogen gas produced when 14.0 moles of hcl is added to an excess amount of magnesium.
Answers: 3

Chemistry, 21.06.2019 18:40
Determine the mass of fuel required for the expected energy consumption in the united states for the next ten years. energy use per person per year in the united states = 3.5 x 1011joules base calculations on current population of 310,000,000.
Answers: 2

Chemistry, 22.06.2019 00:00
What stress will shift the following equilibrium system to the left? n2(g) + 3h2(g) ⇌ 2nh3(g) adding more n2(g) adding more nh3(g) increasing the pressure of the system reducing the volume of the container
Answers: 1

Chemistry, 22.06.2019 07:40
22. a flask containing 450 ml of 0.50 m h2so4 was accidentally knocked to the floor. how many grams of nahco, do you need to put on the spill to neutralize the acid according to the following equation: h2so4(aq)+2 nahcos(aq) na,so(aq) +2 h20()+2 co2(g) d) 38 g a) 2.3 g b) 9.5 g c) 19 g
Answers: 1
You know the right answer?
Determine the empirical formula for a compound that contains c, h and o. it contains 52.14% c and 34...
Questions



Biology, 17.04.2020 06:07

Mathematics, 17.04.2020 06:07


Mathematics, 17.04.2020 06:07

Mathematics, 17.04.2020 06:07

History, 17.04.2020 06:07

Mathematics, 17.04.2020 06:07

Mathematics, 17.04.2020 06:07


Biology, 17.04.2020 06:08



Chemistry, 17.04.2020 06:08




Mathematics, 17.04.2020 06:09

History, 17.04.2020 06:09