Using the standard enthalpies of formation, what is the standard enthalpy of reaction?
co(g)...

Chemistry, 05.09.2019 02:30 kaylienguyen
Using the standard enthalpies of formation, what is the standard enthalpy of reaction?
co(g)+h2o(g)⟶co2(g)+h2(g)
the formation values are as follows:
δhf (co(g)) = −110.525kj/mol
δhf (co2(g)) = −393.509kj/mol
δhf (h2o(g)) = −241.818kj/mol
δhf (h2(g)) = 0

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 12:10
|using the periodic tablewarm-upuse the periodic table in the tools bar to answer the following questions.what elemental classification does oxygen belongto? done
Answers: 3

Chemistry, 22.06.2019 22:00
What mass of glucose is produced when 54g of water react with carbon dioxide
Answers: 1

Chemistry, 22.06.2019 22:40
Percent ionization for a weak acid (ha) is determined by the following formula: percent ionization=[ha] ionized[ha] initial×100%for strong acids, ionization is nearly complete (100%) at most concentrations. however, for weak acids, the percent ionization changes significantly with concentration. the more diluted the acid is, the greater percent ionization.a certain weak acid, ha, has a ka value of 9.4×10? 7.part acalculate the percent ionization of ha in a 0.10 m solution.part bcalculate the percent ionization of ha in a 0.010 m solution
Answers: 1
You know the right answer?
Questions



Computers and Technology, 29.12.2019 01:31

Spanish, 29.12.2019 01:31


Computers and Technology, 29.12.2019 01:31


Mathematics, 29.12.2019 01:31

English, 29.12.2019 01:31




Mathematics, 29.12.2019 01:31


Mathematics, 29.12.2019 01:31

Mathematics, 29.12.2019 01:31

History, 29.12.2019 01:31

Mathematics, 29.12.2019 01:31


![\sum [n_{i}\times \Delta H_{f}(product)_{i}]-\sum [n_{j}\times \Delta H_{f}(reactant)_{j}]](/tpl/images/0223/2160/eef77.png)
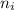 and
and 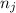 are number of moles of i-th product and j-th reactant in balanced reaction respectively.
are number of moles of i-th product and j-th reactant in balanced reaction respectively.![[(1\times \Delta H_{f}(CO_{2})_{g})]+[(1\times \Delta H_{f}(H_{2})_{g})]-[(1\times \Delta H_{f}(CO)_{g})]-[(1\times \Delta H_{f}(H_{2}O)_{g})]](/tpl/images/0223/2160/f000a.png)
![[(1\times -393.509]+[(1\times 0]-[(1\times -110.525]-[(1\times -241.818]](/tpl/images/0223/2160/cf695.png) kJ = -41.166 kJ
kJ = -41.166 kJ

