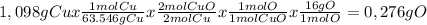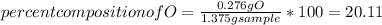Chemistry, 05.09.2019 16:10 alyssatamayo641
When 1.375 g of copper (ii) oxide is reduced on heating in a current of hydrogen, the weight of copper remaining after the reaction is complete is 1.098 g. what is the percent composition of oxygen?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 12:30
Acontrol during an experiment. might change remains constant does not exist does change
Answers: 1

Chemistry, 22.06.2019 17:00
The atoms of a solid aluminum can are close together, vibrating in a rigid structure. if the can is warmed up on a hot plate, what happens to the atoms?
Answers: 1

Chemistry, 22.06.2019 19:00
What is the compound name for the formula [ru(en)2cl2]2+ and [co(en)cl2br]-
Answers: 1

Chemistry, 22.06.2019 22:30
[ou.03jthe pictures below show the wavelengths and intensities of electromagnetic radiations emitted by three stars, star 1, star 2, and star 3. intensity intensity- intensity- 1000 3500 6000 8500 11000 wavelength (a) star 1 1000 3500 6000 8500 11000 1000 3500 6000 8500 11000 wavelength (a) wavelength (a) star 2 star 3 which of these statements is correct about the color of the three stars? star 2 is white in color o star 2 is yellow in color star 1 and star 3 are yellow in color star 1 and star 3 are white in color
Answers: 1
You know the right answer?
When 1.375 g of copper (ii) oxide is reduced on heating in a current of hydrogen, the weight of copp...
Questions

Mathematics, 10.03.2021 04:50



English, 10.03.2021 04:50

Mathematics, 10.03.2021 04:50

Biology, 10.03.2021 04:50


Health, 10.03.2021 04:50


Mathematics, 10.03.2021 04:50

Mathematics, 10.03.2021 04:50

Mathematics, 10.03.2021 04:50

Biology, 10.03.2021 04:50

Spanish, 10.03.2021 04:50


Chemistry, 10.03.2021 04:50

Mathematics, 10.03.2021 04:50


Mathematics, 10.03.2021 04:50

Computers and Technology, 10.03.2021 04:50