
Chemistry, 05.09.2019 16:10 dorothybean
The combustion of propane (c3h8) produces co2 and h2o: c3h8(g) 5o2(g)→3co2(g) 4h2o(g) the reaction of 2.5 mol of o2 with 4.6 mol of c3h8 will produce mol of h2o.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Ionic compounds are made of ions, and yet the overall charge of an ionic compound is neutral. why?
Answers: 1

Chemistry, 22.06.2019 04:20
Which of the following is true for the actual yield of a reaction? it is always calculated as a ratio. it is the yield from the excess reactant. it is the yield from the limiting reactant. it is always less than the theoretical yield.
Answers: 1

Chemistry, 22.06.2019 05:30
What royal scientist used the 29th day of frozen vapor to encounter elements for mastering new culinary creations?
Answers: 1

Chemistry, 22.06.2019 17:30
Aroller coaster is traveling at 13 mi./s when you purchase a hill that is 400 m long and down the hill exonerate at 4.0 m/s squared what is the final velocity of the posterior found your answer to the nearest number
Answers: 1
You know the right answer?
The combustion of propane (c3h8) produces co2 and h2o: c3h8(g) 5o2(g)→3co2(g) 4h2o(g) the reaction...
Questions

Computers and Technology, 14.06.2021 19:00

Mathematics, 14.06.2021 19:00

Mathematics, 14.06.2021 19:00

Mathematics, 14.06.2021 19:00

Mathematics, 14.06.2021 19:00

Mathematics, 14.06.2021 19:00


Mathematics, 14.06.2021 19:00

Business, 14.06.2021 19:00


Mathematics, 14.06.2021 19:00


Chemistry, 14.06.2021 19:00


Computers and Technology, 14.06.2021 19:00





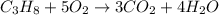
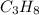 = 4.6
= 4.6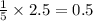 moles of oxygen
moles of oxygen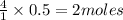 of water
of water

