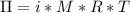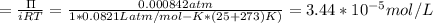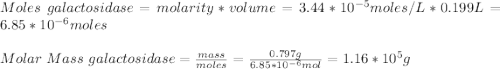Chemistry, 05.09.2019 16:10 elizabethseoane1829
A0.797 g sample of β‑galactosidase is dissolved in water to make 0.199 l of solution, and the osmotic pressure of the solution at 25 ∘c is found to be 0.853 mbar. calculate the molecular mass of β‑galactosidase.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 17:30
You are performing an experiment in a lab to attempt a new method of producing pure elements from compounds. the only problem is that you do not know what element will form. by your previous calculations you know that you will have 6.3 moles of product. when it is complete, you weigh it and determine you have 604.4 grams. what element have you produced?
Answers: 1

Chemistry, 22.06.2019 09:00
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1

Chemistry, 22.06.2019 13:00
12. calculate the hydroxide ion concentration of a solution with ph = 3.25. show all calculations leading to an answer
Answers: 3

Chemistry, 22.06.2019 16:30
An atom with 7 protons, 6 neutrons, and 7 electrons has an atomic mass of amu. (enter a whole number.) numerical answers expected! answer for blank 1:
Answers: 3
You know the right answer?
A0.797 g sample of β‑galactosidase is dissolved in water to make 0.199 l of solution, and the osmoti...
Questions



English, 28.11.2020 19:20

Biology, 28.11.2020 19:20





Mathematics, 28.11.2020 19:20

Mathematics, 28.11.2020 19:20

Business, 28.11.2020 19:20



Spanish, 28.11.2020 19:20

Health, 28.11.2020 19:20

Mathematics, 28.11.2020 19:20




Social Studies, 28.11.2020 19:20