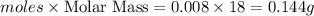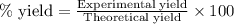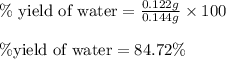Chemistry, 05.09.2019 16:20 kaymillsaps
Gaseous methane (ch4) reacts with gaseous oxygen gas (o2) to produce gaseous carbon dioxide (co2) and gaseous water (h2o) . if 0.122g of water is produced from the reaction of 0.16g of methane and 0.25g of oxygen gas, calculate the percent yield of water.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 02:00
For each of the following types of reactions, write a general reaction formula in the symbolic form—for example, a + b → ab. single-displacement double-displacement synthesis decomposition
Answers: 1

Chemistry, 22.06.2019 14:30
Which of the following represents the ester functional group? a. -coo- b. -cho c. -cooh d. c=o
Answers: 1

Chemistry, 22.06.2019 20:00
If one fission reaction of a uranium-235 atom produced two neutrons, how many neutrons would be released if the chain reaction occurred three more times?
Answers: 1

Chemistry, 23.06.2019 05:30
Scientist think that animals with remarkably similar embryo development probably shared a common ancestor
Answers: 1
You know the right answer?
Gaseous methane (ch4) reacts with gaseous oxygen gas (o2) to produce gaseous carbon dioxide (co2) an...
Questions

Mathematics, 06.08.2019 10:10

History, 06.08.2019 10:10






Mathematics, 06.08.2019 10:10

Mathematics, 06.08.2019 10:10

Mathematics, 06.08.2019 10:10




Mathematics, 06.08.2019 10:10

History, 06.08.2019 10:10



Biology, 06.08.2019 10:10

Chemistry, 06.08.2019 10:10

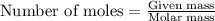 .....(1)
.....(1)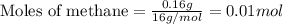
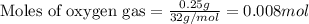
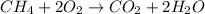
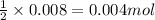 of methane
of methane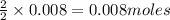 of water
of water