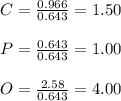Measurements show that unknown compound has the following composition: element mass 38.7 % calcium, 19.9 % phosphorus and 41.2 % oxygen. write the empirical chemical formula of this compound?
(a) ca2po4
(b) ca3po6
(c) ca4p2o4
(d) ca3p2o8 (or ca3(po4)2)
(e) capo4

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 07:00
How many moles are in 7.2 x 10^23 carbon molecules? (*round to the nearest hundredth and include the unit "mol c" after your number) question 6 options:
Answers: 2

Chemistry, 22.06.2019 07:30
Which of the following best supports the concept that genetic information is passed on to offspring from both of their parents, not just one?
Answers: 2

Chemistry, 22.06.2019 07:40
The formation of a solid, also known as a is an indication of a chemical change. precipitate particulate particle powder
Answers: 3

Chemistry, 22.06.2019 09:00
Given the following reaction: c3h8+5o2=3co2+4h20 how many grams of co2 will be produced 7 g of c3h8 and 98 g of o2
Answers: 1
You know the right answer?
Measurements show that unknown compound has the following composition: element mass 38.7 % calcium,...
Questions

English, 25.08.2020 06:01

Chemistry, 25.08.2020 06:01


Physics, 25.08.2020 06:01


Mathematics, 25.08.2020 06:01



Mathematics, 25.08.2020 06:01

Mathematics, 25.08.2020 06:01



Computers and Technology, 25.08.2020 06:01

Mathematics, 25.08.2020 06:01


Mathematics, 25.08.2020 06:01


English, 25.08.2020 06:01

Biology, 25.08.2020 06:01

Mathematics, 25.08.2020 06:01