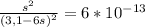Chemistry, 05.09.2019 17:10 wheeler2455
Nickel (ii) ions form a complex ion in the presence of ammonia with a formation constant (kf) of 2.0×10^8:
ni2+ + 6nh3 ⇌ [ni(nh3)6]2+
calculate the molar solubility of nis in 3.1 m nh3. g

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 08:00
Which of the following observations indicates that there is a small, dense, positively charged part in the center of an atom? some uncharged particles are scattered by a gold foil. all uncharged particles are attracted towards a gold foil. all positively charged particles pass straight through a gold foil. some positively charged particles bounce back from a gold foil.
Answers: 2

Chemistry, 22.06.2019 08:30
Which change in temperature is the smallest? a change of 1 thomson degree a change of 1 kelvin degree a change of 1 fahrenheit degree a change of 1 celsius degree
Answers: 1

Chemistry, 22.06.2019 08:40
Write the formula for the following chemicals. 7. e. trinitrogen tetraoxide a calcium phosphate f. magnesium acetate b. potassium sulfide g nickel(iii) cyanide c carbon dioxide h. silver sulfate d. cobalt(ii) chloride
Answers: 1

Chemistry, 22.06.2019 10:30
Asample of air with a volume of 2.20m3 at a pressure of 105 kpa and a temperature of 30c is cooled to 10c and the pressure is reduced to 75.0 kpa. what is the new volume? 6.9 1.34 2.56 43.0 2.88
Answers: 1
You know the right answer?
Nickel (ii) ions form a complex ion in the presence of ammonia with a formation constant (kf) of 2.0...
Questions

Geography, 18.10.2020 16:01

Computers and Technology, 18.10.2020 16:01

Mathematics, 18.10.2020 16:01



Mathematics, 18.10.2020 16:01

Mathematics, 18.10.2020 16:01

English, 18.10.2020 16:01







Geography, 18.10.2020 16:01

Geography, 18.10.2020 16:01


History, 18.10.2020 16:01


English, 18.10.2020 16:01

![\frac{[S^{2-}][Ni(NH3)6^{+2}]}{[NH3]^{6}}](/tpl/images/0223/5248/f04d5.png)