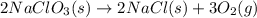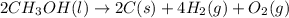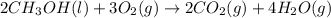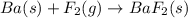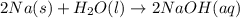Chemistry, 05.09.2019 17:20 skatingby8910
Classify each of the following reactions: i. decopositionii. combinationiii. combustion a. 2ch3oh(l)+3o2(g)→2co2(g)+4h2o(g) b. 2naclo3(s)→2nacl(s)+3o2(g) c. ba(s)+f2(g)→baf2(s) d. 2na(s)+h2o(l)→2naoh(aq) e. 2ch3oh(l)→2c(s)+4h2(g)+o2(g)

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 02:10
26. of of (aq) by (aq) is . if 50.00 ml of 1.05 m is to 25.00 ml of 1.86 m ,at be? ( no is toina of aof) , h.. (p. ). . .
Answers: 3

Chemistry, 22.06.2019 02:10
Determine the percent sulfuric acid by mass of a 1.61 m aqueous solution of h2so4. %
Answers: 2

Chemistry, 22.06.2019 19:30
To calculate percent by mass, use the equation below: calculate the percent by mass of each element. %n = % %h = % %o = %
Answers: 3

Chemistry, 22.06.2019 23:30
If maltose undergoes hydrolysis what subunits does it results to?
Answers: 2
You know the right answer?
Classify each of the following reactions: i. decopositionii. combinationiii. combustion a. 2ch3oh(l)...
Questions






Geography, 16.11.2020 05:10


Mathematics, 16.11.2020 05:10


English, 16.11.2020 05:10


Mathematics, 16.11.2020 05:10




History, 16.11.2020 05:10

Mathematics, 16.11.2020 05:10

Mathematics, 16.11.2020 05:10


Mathematics, 16.11.2020 05:10