
Chemistry, 05.09.2019 17:30 bairdmatthew43
Solute s has a partition coefficient of 6.2 between water (phase 1) and hexane (phase 2). 74.0 ml solution of s in water is extracted six times with 17.0 ml of hexane. calculate the fraction of s remaining in the aqueous phase.

Answers: 1
Another question on Chemistry



Chemistry, 23.06.2019 03:30
Why do electrons further from the nucleus have more energy
Answers: 1

Chemistry, 23.06.2019 07:10
Which one of the following is an oxidation-reduction reaction? naoh + hno3 --> h2o + kno3 naoh + hno3 --> h2o + kno3 so3 + h2o --> h2so4 cacl2 + na2co3 --> caco3 + 2 nacl ch4 + 2 o2 --> co2 + 2 h2o al2(so4)3 + 6 koh --> 2 al(oh)3 + 3 k2so4
Answers: 3
You know the right answer?
Solute s has a partition coefficient of 6.2 between water (phase 1) and hexane (phase 2). 74.0 ml so...
Questions


Mathematics, 29.10.2020 21:50


Mathematics, 29.10.2020 21:50




Mathematics, 29.10.2020 21:50

Social Studies, 29.10.2020 21:50

SAT, 29.10.2020 21:50




English, 29.10.2020 21:50

English, 29.10.2020 21:50

English, 29.10.2020 21:50

English, 29.10.2020 21:50

English, 29.10.2020 21:50

Mathematics, 29.10.2020 21:50

Geography, 29.10.2020 21:50

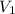 = 74.0 mL
= 74.0 mL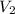 = 17.0 mL
= 17.0 mL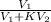
![[\frac{V_{1}}{V_{1} + KV_{2}}]^{3}](/tpl/images/0223/5422/324f2.png)
![[\frac{74.0 ml}{74.0 ml + 6.2 \times 17.0 ml}]^{3}](/tpl/images/0223/5422/2c23d.png)
![[\frac{74.0 ml}{179.4 ml}]^{3}](/tpl/images/0223/5422/f6b3c.png)


