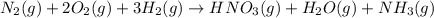Chemistry, 05.09.2019 18:30 itzdryoshi
Nitric acid is often manufactured from the atmospheric gases nitrogen and oxygen, plus hydrogen prepared by reforming of natural gas, in a two-step process. in the first step, nitrogen and hydrogen react to form ammonia: n2(g) + 3h2(g) > 2nh3(g) in the second step, ammonia and oxygen react to form nitric acid and water: nh3(g) + 2o2(g) > hno3(g) + h2o(g) write the net chemical equation for the production of nitric acid from nitrogen, hydrogen and oxygen. be sure your equation is balanced.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 09:20
Give the orbital configuration of the phosphorus (p) atom.
Answers: 1

Chemistry, 22.06.2019 20:00
How are the terms group and period used on the periodic table
Answers: 1

Chemistry, 22.06.2019 23:30
The ammonia molecule in the diagram has the observed bond orientation because
Answers: 1

Chemistry, 23.06.2019 00:00
How many atoms or molecules are there in a mole of a substance?
Answers: 1
You know the right answer?
Nitric acid is often manufactured from the atmospheric gases nitrogen and oxygen, plus hydrogen prep...
Questions

Mathematics, 17.09.2020 14:01

Physics, 17.09.2020 14:01

Mathematics, 17.09.2020 14:01

Mathematics, 17.09.2020 14:01

English, 17.09.2020 14:01

Mathematics, 17.09.2020 14:01

Mathematics, 17.09.2020 14:01

Mathematics, 17.09.2020 14:01

Mathematics, 17.09.2020 14:01

Mathematics, 17.09.2020 14:01

Mathematics, 17.09.2020 14:01

Mathematics, 17.09.2020 14:01

Mathematics, 17.09.2020 14:01

Mathematics, 17.09.2020 14:01

Mathematics, 17.09.2020 14:01

Mathematics, 17.09.2020 14:01

Mathematics, 17.09.2020 14:01

Biology, 17.09.2020 14:01

Mathematics, 17.09.2020 14:01

Mathematics, 17.09.2020 14:01

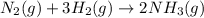 ......(1)
......(1)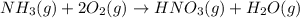 .....(2)
.....(2)