Answers: 1
Another question on Chemistry

Chemistry, 23.06.2019 00:00
#7 how does the structure of amino acids allow them to form a polypeptide? each amino acid has an amino group and a carboxyl group. each amino acid has a hydrogen atom and a carboxyl group. each amino acid has a carboxyl group and an r group. each amino acid has an r group and a hydrogen atom.
Answers: 1

Chemistry, 23.06.2019 02:00
What can be done to make a solid solute dissolve faster in a liquid solvent?
Answers: 1

Chemistry, 23.06.2019 04:00
What is the volume of 2.5 moles of nitrogen gas (n2)at standard temperature and pressure (stp)?
Answers: 1

Chemistry, 23.06.2019 06:30
The molar mass of cu is 63.55 g/mol. the number of grams of cu produced in this reaction is
Answers: 3
You know the right answer?
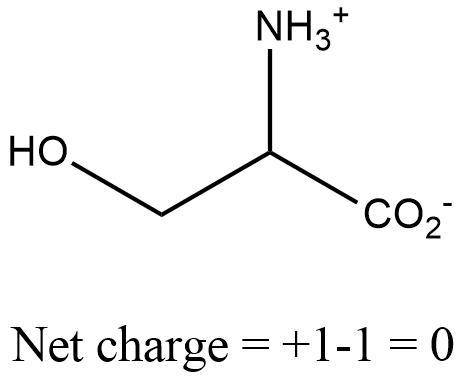
The pka of the α‑carboxyl group of serine is 2.21 , and the pka of its α‑amino group is 9.15 . calcu...
Questions



Physics, 03.12.2021 22:20






Mathematics, 03.12.2021 22:20


History, 03.12.2021 22:20

Mathematics, 03.12.2021 22:20







English, 03.12.2021 22:20

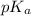 , carboxyl group exists as
, carboxyl group exists as 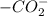 (charged)At pH <
(charged)At pH < 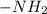 (neutral)At pH <
(neutral)At pH < 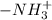 (charged)So, at pH = 8.30, both carboxyl and amino group exists in charged state.Net charge in serine at pH equal to 8.30 is "0".Structure of serine at pH equal to 8.30 has been shown below.
(charged)So, at pH = 8.30, both carboxyl and amino group exists in charged state.Net charge in serine at pH equal to 8.30 is "0".Structure of serine at pH equal to 8.30 has been shown below.