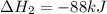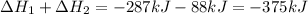Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 06:00
This flow chart shows the amount of energy that is emitted by each type of light. ultraviolet > blue light > yellow light > red light (maximum energy) (minimum energy) in an experiment, shining which type of light on a strip of metal would be least likely to produce the photoelectric effect? ultraviolet light dim blue light bright red light bright yellow light
Answers: 2

Chemistry, 22.06.2019 11:30
Determine the reaction and balance the following equations urgent due in the morning
Answers: 2

Chemistry, 22.06.2019 14:00
The content of manganese (mn) in steel was determined spectrophotometrically and with the use of the standard addition method. an unknown sample of mn from a digested steel sample gave an absorbance of 0.185 when analyzed spectrophotometrically. when 5.00 ml of solution containing 95.5 ppm mn was added to 50.0 ml of the unknown steel solution (digested sample), the absorbance was 0.248. calculate the concentration, in parts-per-million (ppm), of mn in the digested steel sample solution.
Answers: 3

Chemistry, 22.06.2019 19:30
Draw the lewis structure for the trisulfur s3 molecule. be sure to include all resonance structures that satisfy the octet rule.
Answers: 3
You know the right answer?
Calculate δh⁰298 (in kj) for the process p(s) + 5/2 cl2(g) → pcl5(g) from the following information....
Questions

Biology, 14.12.2021 23:00

Social Studies, 14.12.2021 23:00






Mathematics, 14.12.2021 23:00




Mathematics, 14.12.2021 23:00


Mathematics, 14.12.2021 23:00



Mathematics, 14.12.2021 23:00

Mathematics, 14.12.2021 23:00


Mathematics, 14.12.2021 23:00

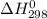 for the process is -375 kJ
for the process is -375 kJ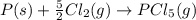 .
.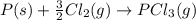 ......
......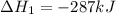
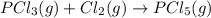 ......
......